Accuracy Check For Infusion Pump With IDA4 White Paper
Accuracy check for infusion pumps using IDA 4 plus
Written by Ichiro Suzuki. Sendai Medical Center, Sendai, Japan
Introduction
 As per the revised Pharmaceutical Affairs Law announced in July 2004, infusion and syringe pumps are classified as specially controlled medical devices. In our hospital, all infusion pumps used in the hospital have been centrally managed in the Department of Clinical Engineering and periodically maintained and inspected by four internal clinical engineers since several years before the revision of the Pharmaceutical Affairs Law (Fig. 1). Since June 2008, accuracy checks have been performed monthly using Fluke Biomedical IDA 4 Plus infusion device analyzers. This article will report the inspection methods for infusion pumps and the failure rate during the period from June 2008 to May 2010 in the hospital as well as the assessment regarding the effects of accuracy checks for infusion and syringe pumps on other clinical operations.
As per the revised Pharmaceutical Affairs Law announced in July 2004, infusion and syringe pumps are classified as specially controlled medical devices. In our hospital, all infusion pumps used in the hospital have been centrally managed in the Department of Clinical Engineering and periodically maintained and inspected by four internal clinical engineers since several years before the revision of the Pharmaceutical Affairs Law (Fig. 1). Since June 2008, accuracy checks have been performed monthly using Fluke Biomedical IDA 4 Plus infusion device analyzers. This article will report the inspection methods for infusion pumps and the failure rate during the period from June 2008 to May 2010 in the hospital as well as the assessment regarding the effects of accuracy checks for infusion and syringe pumps on other clinical operations.
Materials
 Our hospital has 140 infusion pumps in four models, including STC-508 (8 pumps), TE-112 (85), TE-131 (3), and TE-161 (44) (all by TERUMO CORPORATION), and 83 syringe pumps in seven models including STC-525 (13 pumps), TE-312 (8), TE-331 (30), TE-332 (18), TE-352 (9), TE-361 (2), and TE-371 (3) (all by TERUMO CORPORATION).
Our hospital has 140 infusion pumps in four models, including STC-508 (8 pumps), TE-112 (85), TE-131 (3), and TE-161 (44) (all by TERUMO CORPORATION), and 83 syringe pumps in seven models including STC-525 (13 pumps), TE-312 (8), TE-331 (30), TE-332 (18), TE-352 (9), TE-361 (2), and TE-371 (3) (all by TERUMO CORPORATION).
Inspection methods
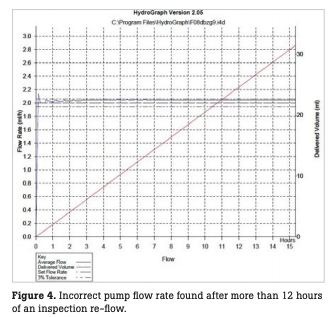
In our hospital, accuracy checks are performed every month for all infusion and syringe pumps, in principle, using two Fluke Biomedical IDA 4 Plus infusion device analyzers and distilled water (Fig. 2). For accuracy checks, the flow rate is measured over three to four hours for each pump with a flow accuracy of 20 mL ± 10 % for infusion pumps and 2 mL ± 3 % for syringe pumps (Fig. 3). For any infusion pump with incorrect values, the test is repeated over 12 hours or more to confirm the flow rate (Fig. 4).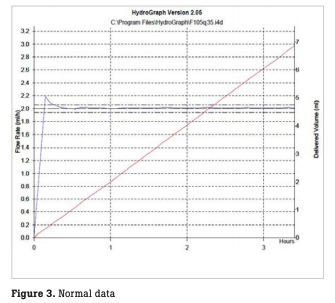 Upon completion of the inspection, a flow rate inspection report is printed out and filed in the equipment control register. All inspected infusion and syringe pumps are marked with a sticker showing inspection completion in monthly different colors and are then available for rent.
Upon completion of the inspection, a flow rate inspection report is printed out and filed in the equipment control register. All inspected infusion and syringe pumps are marked with a sticker showing inspection completion in monthly different colors and are then available for rent.
Results
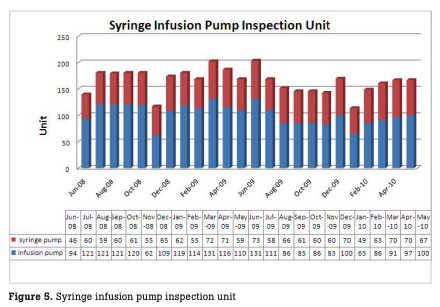
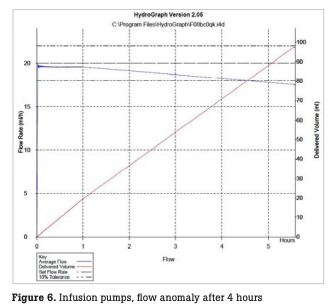
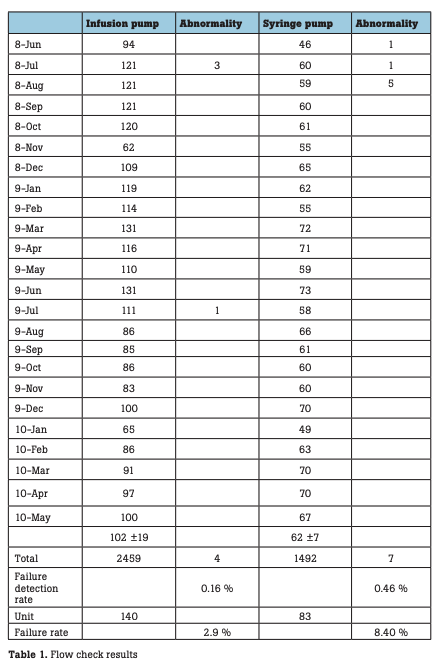
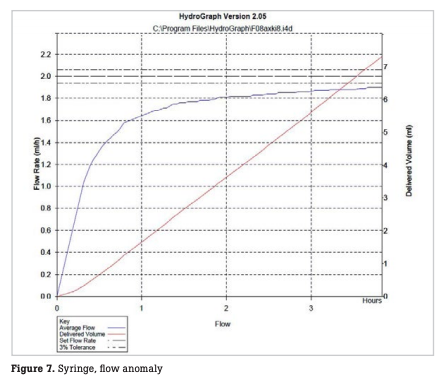 The number of infusion and syringe pumps inspected every month is shown in Fig. 5. During the period, a total of 2,459 infusion pumps were inspected, with a monthly mean of 102 ± 9 pumps. In this period, incorrect flow rates were observed with 4 infusion pumps, resulting in a failure detection rate of 0.16 % relative to the total number of pumps inspected (hereinafter referred to as the failure detection rate) and a failure rate of 2.9 % relative to the number of pumps possessed (hereinafter referred to as the failure rate). During the period, a total of 1,492 syringe pumps were inspected, with a monthly mean of 62 ± 7 pumps. In this period, abnormal flow rates were observed with seven syringe pumps, resulting in a failure detection rate of 0.46 % and a failure rate of 8.4 % (Table 1). In one infusion pump with abnormal flow rates, the flow rate was stable until one hour after the start of measurement, but thereafter began to decrease gradually, deviating from a normal range four hours after the start of measurement (Fig. 6). In some syringe pumps, abnormal flow rates were observed immediately after the start of measurement (Fig. 7) or three hours after the start of measurement (Fig. 8).
The number of infusion and syringe pumps inspected every month is shown in Fig. 5. During the period, a total of 2,459 infusion pumps were inspected, with a monthly mean of 102 ± 9 pumps. In this period, incorrect flow rates were observed with 4 infusion pumps, resulting in a failure detection rate of 0.16 % relative to the total number of pumps inspected (hereinafter referred to as the failure detection rate) and a failure rate of 2.9 % relative to the number of pumps possessed (hereinafter referred to as the failure rate). During the period, a total of 1,492 syringe pumps were inspected, with a monthly mean of 62 ± 7 pumps. In this period, abnormal flow rates were observed with seven syringe pumps, resulting in a failure detection rate of 0.46 % and a failure rate of 8.4 % (Table 1). In one infusion pump with abnormal flow rates, the flow rate was stable until one hour after the start of measurement, but thereafter began to decrease gradually, deviating from a normal range four hours after the start of measurement (Fig. 6). In some syringe pumps, abnormal flow rates were observed immediately after the start of measurement (Fig. 7) or three hours after the start of measurement (Fig. 8).
Discussion
One infusion pump had abnormal flow rates four hours after the start of measurement. Similarly, one syringe pump had incorrect flow rates three hours after the start of measurement, suggesting it may be impossible to ensure reliable accuracy control by approximately one hour of measurement. Accuracy checks may require measurement over three to four hours as is performed in our hospital. On the other hand, four clinical engineers in our hospital are engaged in multiple clinical operations, and any of them cannot be exclusively committed to inspection duties. If the inspection takes one hour, they cannot concentrate on the other clinical operations.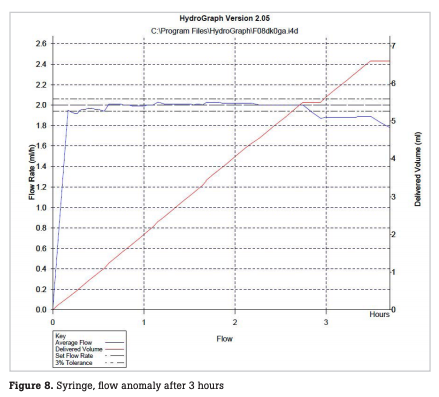 A Fluke Biomedical IDA 4 Plus infusion device analyzer, which allows several hours of measurement without supervision, sets them free from attendance for the flow rate measurement and enables them to perform other clinical operations instead. Using IDA 4 Plus, which allows accuracy check for four infusion pumps in parallel at once, accuracy checks can be performed for up to 16 pumps per day in our hospital. The flow rate inspection is associated with the following problems. First, inspection of pumps must be carried out with due attention to the status of use because the pumps cannot be available to use during several hours of inspection. Secondly, the results of flow rate inspection are currently recorded and stored on a paper basis. Given a huge amount of printouts to be output in the future, it will be more desirable to record and store the results electronically rather than on a paper basis. Currently in Japan, however, regulatory audit of hospitals requires paper-based disclosure of data, warranting future studies on the validity of electronic recording and storing.
A Fluke Biomedical IDA 4 Plus infusion device analyzer, which allows several hours of measurement without supervision, sets them free from attendance for the flow rate measurement and enables them to perform other clinical operations instead. Using IDA 4 Plus, which allows accuracy check for four infusion pumps in parallel at once, accuracy checks can be performed for up to 16 pumps per day in our hospital. The flow rate inspection is associated with the following problems. First, inspection of pumps must be carried out with due attention to the status of use because the pumps cannot be available to use during several hours of inspection. Secondly, the results of flow rate inspection are currently recorded and stored on a paper basis. Given a huge amount of printouts to be output in the future, it will be more desirable to record and store the results electronically rather than on a paper basis. Currently in Japan, however, regulatory audit of hospitals requires paper-based disclosure of data, warranting future studies on the validity of electronic recording and storing.
Conclusion
Since short duration of flow rate inspection has problems with accuracy control, at least three hours of flow rate inspection is desirable. Introduction of Fluke Biomedical IDA 4 Plus infusion device analyzers has led to an increase in the failure detection rate of infusion and syringe pumps and an improvement in safety. It may also contribute to more efficient operations by the clinical engineers. IDA 4 Plus is expected to reduce the duration of inspection and improve productivity because it ensures the inspection of flow rate with high accuracy and allows simultaneous measurements at multiple channels and electronic recording and storing with minimum clinical engineers attendance. The “oven” is set to a temperature between 28 °C and 40 °C. The Skin Temperature Sensor is plugged into the temperature control module and the temperature sensing element is placed in the “oven”. The difference in temperature between the “oven” and the sensor should not exceed 0.3 °C.
 About the author
About the author
Mr. Ichiro Suzuki is the Chairman of Miyagi Association of Clinical Engineering Technologists. He is currently the Chief Perfusionist and Clinical Engineer at the Sendai Medical Center, Sendai, Japan. Mr. Suzuki is an expert in his field with extensive practical and teaching experience in clinical engineering. He is a Clinical Instructor at the College of Engineering in Tohoku Bunka Gakuen. In addition to his current professional responsibilities, he is pursuing a Ph.D, on Artificial Organs from the Department of Biomedical Engineering, Tohoku University Graduate School of Biomedical Engineering.
